The Autonomous Loop: How Agentic AI is Unifying Drug Discovery and Supply Chain in 2026
The Autonomous Loop: How Agentic AI is Unifying Drug Discovery and Supply Chain in 2026
The Rise of the "Doer" Agents
For the last five years, digital transformation in pharma was defined by predictive analytics—forecasts that told us when a shipment might be delayed or which molecule might work. Today, that paradigm is obsolete. The defining technology of 2026 is Agentic AI: autonomous software agents capable of executing complex workflows without human intervention.
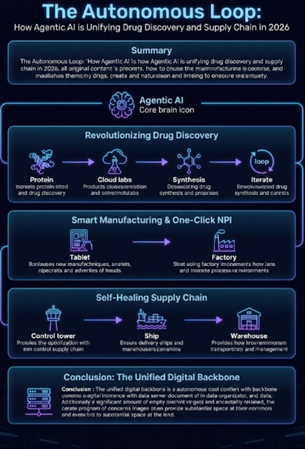
Revolutionizing Drug Discovery: From In-Silico to Wet Lab
The impact of this shift on R&D is profound. We are moving from computer-aided drug design (CADD) to Generative Biology. In 2026, AI models are no longer limited to screening existing libraries; they are designing novel protein structures and small molecules from scratch, tailored to specific genetic profiles.
Most critically, these digital minds are now connected to physical "cloud labs." An AI agent can now design a molecule, order its synthesis in a robotic wet lab, analyze the mass spectrometry results, and iterate on the design—all in a closed loop. This has compressed hit-to-lead timelines from months to weeks.
The Self-Healing Supply Chain
Perhaps the most immediate ROI for the industry lies in logistics. With geopolitical risks ranking as a top concern for 61% of supply chain leaders, resilience is non-negotiable. Modern Supply Chain Control Towers are now staffed by AI agents that monitor global data streams 24/7.
- Predictive Rerouting: If a port strike is detected in Rotterdam, the agent automatically books alternative freight capacity in Antwerp before a human operator even opens their email.
- Dynamic Inventory: Agents balance stock levels across regional distribution centers in real-time, predicting demand spikes for specific SKUs based on local epidemiological data.
Smart Manufacturing and "One-Click" NPI
The bridge between R&D and Supply Chain is Manufacturing, and here, Agentic AI is solving the notorious "tech transfer" bottleneck. New Product Introduction (NPI) has historically been a manual, error-prone process.
In 2026, we are seeing the emergence of autonomous GMP configuration. AI agents can read the parameters of a new drug formulation and autonomously configure shop floor systems, generating batch records and validation protocols for human review. This "one-click" deployment capability is significantly reducing the time it takes to bring new therapies from the lab bench to the patient.
Conclusion: The Unified Ecosystem
The siloed approach of "R&D AI" versus "Supply Chain AI" is disappearing. The leading companies of 2026 are building a unified digital backbone where data flows seamlessly from discovery to delivery. In this new ecosystem, intelligence is not just an add-on; it is the central nervous system of the pharmaceutical enterprise.
